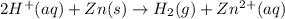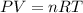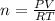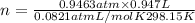Chemistry, 18.10.2019 23:30 makaylahunt
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) .
when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 ∘c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 ∘c .)
what mass of hydrogen gas is collected?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed...
Questions

Biology, 19.08.2019 16:20




Mathematics, 19.08.2019 16:20



Geography, 19.08.2019 16:20

Mathematics, 19.08.2019 16:20


Social Studies, 19.08.2019 16:20

English, 19.08.2019 16:20





Mathematics, 19.08.2019 16:20


History, 19.08.2019 16:20