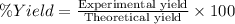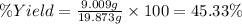Chemistry, 18.10.2019 23:30 blaze9889t
Alum is a compound used in a variety of applications including cosmetics, water purification, and as a food additive. it can be synthesized from aluminum metal, sulfuric acid, water, and potassium hydroxide, as seen in the equation. 2 al(s) 2 koh(aq) 4 h2so4(aq) 10 h2o(l) ⟶ 2 kal(so4)2∙12 h2o(s) 3 h2(g) alum using the data, determine the theoretical and percent yield for this alum synthesis. note that aluminum is the limiting reactant. description mass (g) bottle mass 10.221 bottle mass with aluminum pieces 11.353 final product and bottle mass 19.230 what is the theoretical yield of alum

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Alum is a compound used in a variety of applications including cosmetics, water purification, and as...
Questions






English, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01


French, 12.08.2020 04:01


Mathematics, 12.08.2020 04:01

Biology, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01

History, 12.08.2020 04:01

Health, 12.08.2020 04:01


History, 12.08.2020 04:01


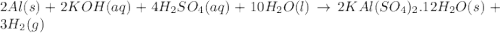
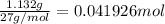
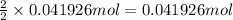 of alum.
of alum.