Chemistry, 19.10.2019 00:10 jimperez9616
During world war ii, a portable source of hydrogen gas was needed for weather balloons, and solid metal hydrides were the most convenient form. many metal hydrides react with water to generate the metal hydroxide and hydrogen. two candidates were lithium hydride and magnesium hydride. what volume of gas is formed from 1.40 lb of each hydride at 750. torr and 27°c?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1

Chemistry, 23.06.2019 14:50
Write an equation to show action of positive and negative catalyst
Answers: 1
You know the right answer?
During world war ii, a portable source of hydrogen gas was needed for weather balloons, and solid me...
Questions

Mathematics, 27.06.2019 04:00


History, 27.06.2019 04:00

Mathematics, 27.06.2019 04:00


Business, 27.06.2019 04:00



Mathematics, 27.06.2019 04:00



Mathematics, 27.06.2019 04:00




Geography, 27.06.2019 04:00

Chemistry, 27.06.2019 04:00



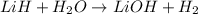
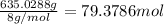
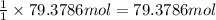 of hydrogen gas.
of hydrogen gas.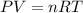
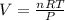
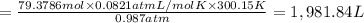
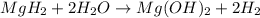
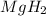 = 1.40 lb = 635.0288 g
= 1.40 lb = 635.0288 g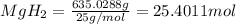
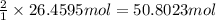 of hydrogen gas.
of hydrogen gas.