
An herbicide contains only c, h, cl, and n. the complete combustion of a 200.0 mg sample of the herbicide in excess oxygen produced 209.2 ml of co2 and 122.0 ml of h2o vapor at stp. a separate analysis determined the 200.0 mg sample contained 55.14 mg cl. determine the percent composition of the herbicide.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
An herbicide contains only c, h, cl, and n. the complete combustion of a 200.0 mg sample of the herb...
Questions


Mathematics, 30.03.2021 16:20



Biology, 30.03.2021 16:20



Biology, 30.03.2021 16:20

Physics, 30.03.2021 16:20




Mathematics, 30.03.2021 16:20


Mathematics, 30.03.2021 16:20

Mathematics, 30.03.2021 16:20



Mathematics, 30.03.2021 16:20

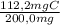 ×100 = 56,1%C
×100 = 56,1%C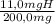 ×100 = 5,5%H
×100 = 5,5%H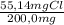 ×100 = 27,6%Cl
×100 = 27,6%Cl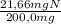 ×100 = 10,8%N
×100 = 10,8%N


