
Chemistry, 19.10.2019 01:00 adiaripley6408
The energy of an electrostatic interaction between two charged atoms is dependent on the charges on the atoms, the distance between them, and the dielectric constant of the solvent. for example, the strength of a weak acid (ka, acid dissociation constant) depends on the strength of the electrostatic interaction between a negatively charged carboxylic acid group and a proton. the solvent dielectric constant has a large influence on the pka for weak acids. select the statements that correctly explain the influence of two solvents, water and hexane, on the pka of acetic acid.
in water, the association of ch3coo– and h will be favored because charge-charge interactions are stronger in water.
nonpolar solvents like hexane will weaken charge-charge interactions resulting in more dissociation of h .
the pka will be higher in hexane.
hexane cannot affect the pka of acetic acid since hexane cannot donate or accept h .
more h will dissociate in water because the dielectric constant is higher.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
The energy of an electrostatic interaction between two charged atoms is dependent on the charges on...
Questions

Mathematics, 01.10.2019 04:00

Mathematics, 01.10.2019 04:00


















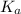 will be low , hence
will be low , hence 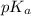 will be high.
will be high. 

