
Chemistry, 19.10.2019 00:30 hannahbannana98
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthalein as the indicator. in the reaction of oxalic acid with naoh, every 1 mole of oxalic acid is deprotonated by 2 moles of naoh. the molecular weight of oxalic acid is 90.03 g/mol and you use 0.60 g in 50 ml water for the titration. you use 15.20 ml of your naoh solution in order to reach the endpoint of the titration. what is the concentration of your naoh solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1


Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthale...
Questions






Chemistry, 12.04.2021 23:00

Biology, 12.04.2021 23:00

Mathematics, 12.04.2021 23:00

Mathematics, 12.04.2021 23:00







English, 12.04.2021 23:00



Business, 12.04.2021 23:00

Mathematics, 12.04.2021 23:00

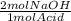 = 0.0133 mol NaOH
= 0.0133 mol NaOH

