
Chemistry, 19.10.2019 00:30 laequity7325
Sodium thiosulfate, na2s2o3, is used as a fixer in photographic film developing. the amount of na2s2o3 in a solution can be determined by a titration with iodine, i2, according to the equation: 2na2s2o3(aq) + i2(aq) --> na2s4o6 +2nai(aq). calculate the concentration of the na2s2o3 solution if 23.50 ml of a 0.1710 m i2 solution react exactly with a 100.0 ml sample of the na2s2o3 solution. use 4 sig. fig. aside, the end of the titration is determined by the color. nai is a pale yellow and i2 is a deep purple. just when the purple color persists, all the iodine that can react has reacted.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Sodium thiosulfate, na2s2o3, is used as a fixer in photographic film developing. the amount of na2s2...
Questions

Physics, 10.07.2019 22:20

Biology, 10.07.2019 22:20



Mathematics, 10.07.2019 22:20



Business, 10.07.2019 22:20






Mathematics, 10.07.2019 22:20

Biology, 10.07.2019 22:20


Chemistry, 10.07.2019 22:20

Mathematics, 10.07.2019 22:30

Mathematics, 10.07.2019 22:30

Mathematics, 10.07.2019 22:30

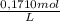 = 4,0185x10⁻³ moles of I₂
= 4,0185x10⁻³ moles of I₂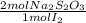 = 8,037x10⁻³ moles of Na₂S₂O₃
= 8,037x10⁻³ moles of Na₂S₂O₃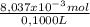 = 0,08037 M
= 0,08037 M

