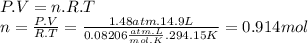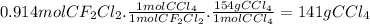Chemistry, 19.10.2019 02:30 Gracebrown6990
Freon−12 (cf2cl2), widely used as a refrigerant and aerosol propellant, is a dangerous air pollutant. in the troposphere, it traps heat 25 times as effectively as co2, and in the stratosphere, it participates in the breakdown of ozone. freon−12 is prepared industrially by reaction of gaseous carbon tetrachloride with hydrogen fluoride. hydrogen chloride gas also forms. how many grams of carbon tetrachloride are required for the production of 14.9 dm3 of freon−12 at 21°c and 1.48 atm?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Freon−12 (cf2cl2), widely used as a refrigerant and aerosol propellant, is a dangerous air pollutant...
Questions


Mathematics, 23.07.2019 20:00


History, 23.07.2019 20:00





Mathematics, 23.07.2019 20:00







Social Studies, 23.07.2019 20:00

English, 23.07.2019 20:00


Physics, 23.07.2019 20:00

Chemistry, 23.07.2019 20:00