
This question has multiple parts.
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with 85.00 g water at an initial temperature 25.00oc. after dissolution of the salt, the final temperature of the calorimeter contents was 23.34oc.
assuming the solution has a heat capacity of 4.18 j/g∙oc, and assuming no heat loss to the calorimeter, calculate the enthalpy of solution (∆hsoln) for the dissolution of nh4no3 in units of kj/mol.
∆ = kj/mol
b. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with 85.00 g water at an initial temperature 25.00oc. after dissolution of the salt, the final temperature of the calorimeter contents was 23.34oc.
if the enthalpy of hydration for nh4no3 is -630. kj/mol, calculate the lattice energy of nh4no3.
lattice energy = kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
This question has multiple parts.
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with...
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with...
Questions


English, 26.12.2021 18:30

Mathematics, 26.12.2021 18:30

Mathematics, 26.12.2021 18:30

Biology, 26.12.2021 18:30



English, 26.12.2021 18:40

Health, 26.12.2021 18:40

English, 26.12.2021 18:40

English, 26.12.2021 18:40

World Languages, 26.12.2021 18:40

Chemistry, 26.12.2021 18:40

Mathematics, 26.12.2021 18:40

Mathematics, 26.12.2021 18:40


Mathematics, 26.12.2021 18:50


Mathematics, 26.12.2021 18:50

English, 26.12.2021 18:50

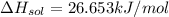

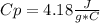
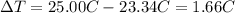
![m=m_{water} + m_{salt]=1.81g +85g= 86.81g](/tpl/images/0333/2995/cfc86.png)
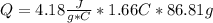
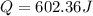
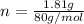
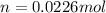
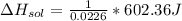
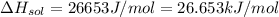
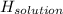 = 26.20 kJ/mol
= 26.20 kJ/mol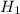 ) = - 656.20 kJ/mol
) = - 656.20 kJ/mol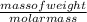
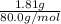

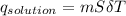
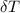 = 25.00°C - 23.34°C
= 25.00°C - 23.34°C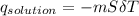 (since heat is lost by the water to the compound)
(since heat is lost by the water to the compound)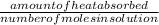
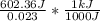
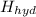 + Δ
+ Δ

