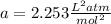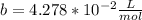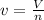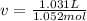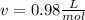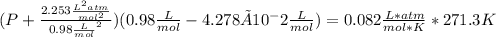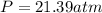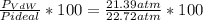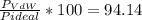1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3 k should exert a pressure of 22.72 atm. by what percent does the pressure calculated using the van der waals' equation differ from the ideal pressure? for ch4 gas,
a = 2.253 l2atm/mol2 and
b = 4.278×10-2 l/mol.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3...
Questions



Computers and Technology, 20.09.2020 14:01



Mathematics, 20.09.2020 14:01



English, 20.09.2020 14:01

History, 20.09.2020 14:01






English, 20.09.2020 14:01

Biology, 20.09.2020 14:01