
Chemistry, 19.10.2019 04:20 viktoria1198zz
If the percent yield for the following reaction is 80.37%, and 63.21 g of no2 are used in the reaction mixture, how many grams of nitric acid, hno3(aq), are produced in the experiment? 3 no2(g) + h2o(l) → 2 hno3(aq) + no(g) molar mass no2 = 46.01 g/mol and hno3 = 63.01 g/mol.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3


Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
If the percent yield for the following reaction is 80.37%, and 63.21 g of no2 are used in the reacti...
Questions

Mathematics, 03.10.2019 01:00




Mathematics, 03.10.2019 01:00





Social Studies, 03.10.2019 01:00


Computers and Technology, 03.10.2019 01:00








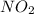 is 46.01 g/mol and mass of
is 46.01 g/mol and mass of 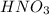 is 63.01 g/mol.
is 63.01 g/mol.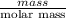
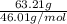
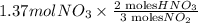
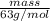
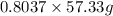
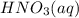 , are produced in the experiment.
, are produced in the experiment.

