
Chemistry, 19.10.2019 04:30 lapointayyy6388
What is the vapor pressure (in kpa) of ethanol, ch3ch2oh, over a solution which is composed of 18.00 ml of ethanol and 12.55 g of benzoic acid, c6h5cooh, at 35ºc ?
enter your number with two digits past the decimal.
•pºethanol at 35ºc = 13.693 kpa
•density of ethanol = 0.789 g/mol, molar mass of ethanol = 46.07
•molar mass of benzoic acid = 122.12 g/mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

You know the right answer?
What is the vapor pressure (in kpa) of ethanol, ch3ch2oh, over a solution which is composed of 18.00...
Questions

Biology, 19.08.2019 14:30

Geography, 19.08.2019 14:30

Mathematics, 19.08.2019 14:30

Geography, 19.08.2019 14:30

Mathematics, 19.08.2019 14:30

Mathematics, 19.08.2019 14:30

Arts, 19.08.2019 14:30

Mathematics, 19.08.2019 14:30


Social Studies, 19.08.2019 14:30

Mathematics, 19.08.2019 14:30


Mathematics, 19.08.2019 14:30




Mathematics, 19.08.2019 14:30

Social Studies, 19.08.2019 14:30


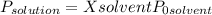 (1)
(1)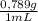 ×
×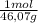 = 0,3083 mol Ethanol.
= 0,3083 mol Ethanol.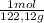 = 0,1028 mol benzoic acid.
= 0,1028 mol benzoic acid.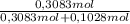 = 0,7499
= 0,7499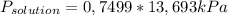 = 10,27 kPa
= 10,27 kPa

