
Chemistry, 19.10.2019 05:30 brianwins07
Consider the following data on some weak acids and weak bases:
acid base
ka name formula kb name formula
6.8x10-4 hydrofluoric acid hf 1.8x10-5 ammonia nh3
4.9x10-10 hydrocyanic acid hcn 1.7x10-9 pyridine c5h5n
use this data to rank the following solutions in order of increasing ph. in other words, select a '1' next to the solution that will have the lowest ph, a '2' next to the solution that will have the next lowest ph, and so on.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Consider the following data on some weak acids and weak bases:
acid base
ka...
acid base
ka...
Questions


Spanish, 11.02.2021 22:30

Mathematics, 11.02.2021 22:30



Mathematics, 11.02.2021 22:30



Mathematics, 11.02.2021 22:30

Biology, 11.02.2021 22:30


Mathematics, 11.02.2021 22:30

English, 11.02.2021 22:30



Physics, 11.02.2021 22:30


Mathematics, 11.02.2021 22:30


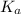 higher strength of acidity because of higher degree of dissociation .
higher strength of acidity because of higher degree of dissociation . 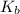 represents higher strength of basicity . The higher the basicity , the lower the acidity .
represents higher strength of basicity . The higher the basicity , the lower the acidity . 

