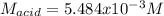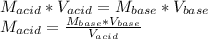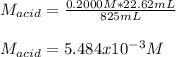Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1

Chemistry, 23.06.2019 21:30
If 1.00 mol cs2 reacts with 1.00 mol o2, identify the limiting reactions
Answers: 3

Chemistry, 24.06.2019 00:00
Which statements correctly match a chemical name with its formula? use the list of polyatomic ions and the periodic table to you answer. the chemical formula for ammonium carbonate is nh4hco3. the chemical formula for ammonium hypochlorite is nh4clo. the chemical formula for ammonium nitrate is nh4no3. the chemical formula for ammonium phosphate is nh4(po4)3. the chemical formula for ammonium sulfate is (nh4)2so3.
Answers: 3
You know the right answer?
825 ml sample of an unknown hclo4 solution requires titration with 22.62 ml of 0.2000 m naoh to reac...
Questions

Social Studies, 31.08.2019 19:20

Mathematics, 31.08.2019 19:20

Advanced Placement (AP), 31.08.2019 19:20


Business, 31.08.2019 19:20

Mathematics, 31.08.2019 19:20

History, 31.08.2019 19:20


History, 31.08.2019 19:20



English, 31.08.2019 19:20

Physics, 31.08.2019 19:20

Mathematics, 31.08.2019 19:20


Law, 31.08.2019 19:20

English, 31.08.2019 19:20

Social Studies, 31.08.2019 19:20