
Chemistry, 21.10.2019 23:30 rachelsweeney10
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then adds iron powder to the conical flask at once. then, she stirs it briskly to initiate and facilitate the reaction between copper sulfate and iron powder. while stirring continuously, vanessa observes that the blue color of copper sulfate solution is fading substantially. moreover, she also observes the deposition of a reddish-brown mass at the bottom of the flask.
finally, a stage is reached where the solution becomes pale green in color. on further stirring of the solution, there is no change in the intensity of color. a distinct reddish-brown layer is deposited in the conical flask. however, on weighing the reaction mixture, she finds that the mass of the reaction mixture remains unchanged at 215.4 g. the reddish-brown layer is then separated from the pale green solution and allowed to dry in an oven. the moisture is completely evaporated, and the dried layer is weighed. its weight is found to be 63.5 g.
what is the mass of required iron powder and the formed iron sulfate in the chemical reaction?
a
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
b
the mass of iron powder required to complete the reaction was 63.5 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
c
the mass of iron powder required to complete the reaction was 159.6 g, while the mass of iron sulfate formed in the reaction was 63.5 g.
d
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 159.6 g.
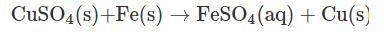

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then...
Questions


Mathematics, 26.02.2021 04:00


Mathematics, 26.02.2021 04:00





History, 26.02.2021 04:00


Business, 26.02.2021 04:00


Mathematics, 26.02.2021 04:00





Mathematics, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00



