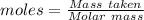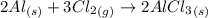Chemistry, 22.10.2019 03:30 carterh166
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(> 2alcl3(s) you are given 18.0g of aluminum and 23.0g of chlorine gas. part a: if you had excess chlorine, how many moles of of aluminum chloride could be produced from 18.0g of aluminum? express your answer numerically in moles. part b: if you had excess aluminum, how many moles of aluminum chloride could be produced from 23.0g of chlorine gas, cl2? express your answer numerically in moles.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2...
Questions

Biology, 10.02.2020 21:28



Biology, 10.02.2020 21:29


Mathematics, 10.02.2020 21:29

Mathematics, 10.02.2020 21:29

Mathematics, 10.02.2020 21:29




Mathematics, 10.02.2020 21:29






Business, 10.02.2020 21:29


Mathematics, 10.02.2020 21:29