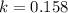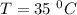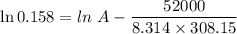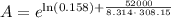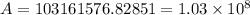Chemistry, 22.10.2019 04:00 Kingdcn6261
For a reaction with an activation energy of 52.0 kilojoules per mole and at a temperature of 35°c find the pre-exponential factor if the rate constant is 0.158 a) 0.161 в) 1.03 x 10% c) 6.42 x 1076 10 d) 2.42 x 10% e) 6.71 x 106

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
For a reaction with an activation energy of 52.0 kilojoules per mole and at a temperature of 35°c fi...
Questions



Computers and Technology, 03.12.2021 21:50

Mathematics, 03.12.2021 21:50



Arts, 03.12.2021 21:50




Mathematics, 03.12.2021 21:50

Social Studies, 03.12.2021 21:50




Biology, 03.12.2021 22:00

Mathematics, 03.12.2021 22:00

Business, 03.12.2021 22:00

Mathematics, 03.12.2021 22:00

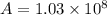
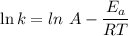
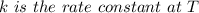
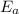 is the activation energy
is the activation energy