
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burned in excess oxygen to yield 4.23 g of co2 and 1.01 g of h2o. another sample of the same compound, of mass 4.14 g, yielded 2.11 g of so3. a third sample, of mass 5.66 g, was burned under different conditions to yield 2.27 g of hno3 as the only nitrogen containing product. determine the empirical formula of the compound.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burn...
Questions

Mathematics, 29.08.2020 04:01



Mathematics, 29.08.2020 04:01

Mathematics, 29.08.2020 04:01

Engineering, 29.08.2020 04:01




Computers and Technology, 29.08.2020 04:01





Mathematics, 29.08.2020 04:01


Biology, 29.08.2020 04:01


Mathematics, 29.08.2020 04:01

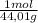 = 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt%
= 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt% 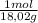 = 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%
= 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%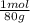 = 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%
= 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%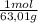 = 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%
= 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%

