Chemistry, 22.10.2019 20:00 destinyaus14
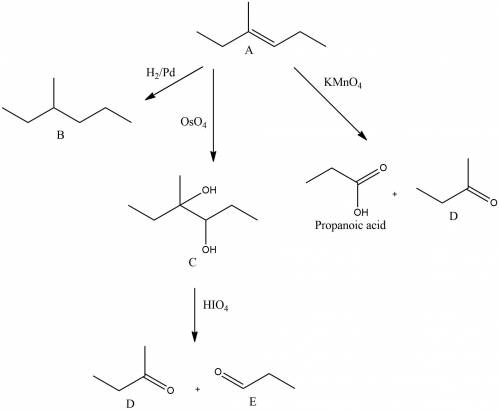
An unknown hydrocarbon a with the formula c7h14 reacts with 1 molar equivalent of h2 over a palladium catalyst to give hydrocarbon b. hydrocarbon a also reacts with oso4 to give diol c. treatment of diol c with periodic acid gives ketone d and aldehyde e. when oxidized with kmno4 in acidic solution, a gives two fragments. one fragment is propionic acid, ch3ch2co2h, and the other fragment is ketone d. draw the structure of compound d.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

You know the right answer?
An unknown hydrocarbon a with the formula c7h14 reacts with 1 molar equivalent of h2 over a palladiu...
Questions


History, 22.04.2020 23:28

Mathematics, 22.04.2020 23:28


Mathematics, 22.04.2020 23:28

Mathematics, 22.04.2020 23:28


English, 22.04.2020 23:28

Mathematics, 22.04.2020 23:28

Chemistry, 22.04.2020 23:28

Mathematics, 22.04.2020 23:28

History, 22.04.2020 23:28


Mathematics, 22.04.2020 23:28



Mathematics, 22.04.2020 23:28

History, 22.04.2020 23:28


Mathematics, 22.04.2020 23:28

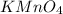 .The hydrocarbon A should contain only one double bond as it reacts with 1 molar equivalent of
.The hydrocarbon A should contain only one double bond as it reacts with 1 molar equivalent of 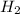 over palladium.The hydrocarbon A contains seven carbon atom. Therefore the ketone D should contain 4 carbon atoms as propionic acid ( 3 carbon atom) is produced as another fragment.Reaction and structure of D has been shown below.
over palladium.The hydrocarbon A contains seven carbon atom. Therefore the ketone D should contain 4 carbon atoms as propionic acid ( 3 carbon atom) is produced as another fragment.Reaction and structure of D has been shown below.