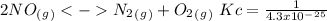Chemistry, 22.10.2019 21:00 kobiemajak
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g) + o2(g) ⇌ 2no(g) kc = 4.3 × 10−25 2no(g) + o2(g) ⇌ 2no2(g) kc = 6.4 × 109 determine the values of the equilibrium constants for the following equations at the same temperature: (a) 4no(g) ⇌ n2(g) + 2no2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2


Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g)...
Questions

Mathematics, 04.11.2020 17:30





Advanced Placement (AP), 04.11.2020 17:30







Mathematics, 04.11.2020 17:30

Mathematics, 04.11.2020 17:30

Health, 04.11.2020 17:30

Social Studies, 04.11.2020 17:30

History, 04.11.2020 17:30


Mathematics, 04.11.2020 17:30

Biology, 04.11.2020 17:30




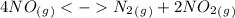
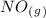 as a reactive in the target reaction and
as a reactive in the target reaction and