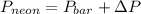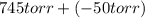Chemistry, 22.10.2019 21:00 joshuasanders7490
Aflask containing neon gas is connected to an open-endedmercury manometer. the open end is exposed to the atmosphere, wherethe prevailing pressure is 745 torr. the mercury level in the openarm is 50 mm below that in the arm connected to the flask of neon. what is the neon pressure, in torr?
a. -50 torr
b. 50 torr
c. 695 torr
d. 795 torr
e. none of these choices is correct.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Aflask containing neon gas is connected to an open-endedmercury manometer. the open end is exposed t...
Questions









Mathematics, 05.05.2020 07:53


Mathematics, 05.05.2020 07:53

Mathematics, 05.05.2020 07:53



History, 05.05.2020 07:53


Mathematics, 05.05.2020 07:53


Mathematics, 05.05.2020 07:53

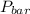 = 745 torr
= 745 torr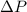 = - 50 (the value is negative because on the open side its level is lower than the side connected to the gas)
= - 50 (the value is negative because on the open side its level is lower than the side connected to the gas)