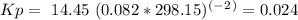Enter your answer in the provided box. a united nations toxicologist studying the properties of mustard gas, s(ch2ch2cl)2, a blistering agent used in warfare, prepares a mixture of 0.675 m scl2and 0.973 m c2h4and allows it to react at room temperature (20.0°c): scl2(g) + 2 c2h4(g) ⇌ s(ch2ch2cl)2(g) at equilibrium,[s( ch2ch2cl)2] = 0.350 m. calculate .

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3


Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Enter your answer in the provided box. a united nations toxicologist studying the properties of must...
Questions


Computers and Technology, 30.09.2019 21:00



Mathematics, 30.09.2019 21:00


Advanced Placement (AP), 30.09.2019 21:00

Mathematics, 30.09.2019 21:00

Mathematics, 30.09.2019 21:00




Physics, 30.09.2019 21:00


Geography, 30.09.2019 21:00

Mathematics, 30.09.2019 21:00



History, 30.09.2019 21:00

History, 30.09.2019 21:00

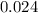
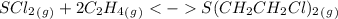
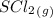 = 0.325 M
= 0.325 M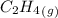 = 0.273 M
= 0.273 M 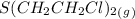 = 0.35 M
= 0.35 M![Kc=\frac{[S(CH_2CH_2Cl)_2]}{[SCl_2][C_2H_4]^2}=\frac{[0.35]}{[0.325][0.273]^2}=14.45](/tpl/images/0341/2750/de285.png)
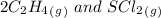 = -2
= -2