Chemistry, 22.10.2019 23:30 hanacat6174
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following data. the heat capacity of the bomb calorimeter is 34.65 kj/k and the combustion of 1.752 g of ethanol raises the temperature of the calorimeter from 294.42 k to 295.92 k .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following...
Questions

Spanish, 12.10.2019 23:30

Biology, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Biology, 12.10.2019 23:30


Chemistry, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30


Social Studies, 12.10.2019 23:30

Computers and Technology, 12.10.2019 23:30


Mathematics, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

History, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30


Mathematics, 12.10.2019 23:30


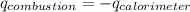
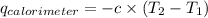
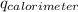 = heat released by calorimeter = ?
= heat released by calorimeter = ?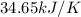
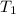 = initial temperature of calorimeter = 294.42 K
= initial temperature of calorimeter = 294.42 K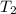 = final temperature of calorimeter = 295.92 K
= final temperature of calorimeter = 295.92 K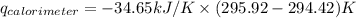
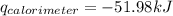
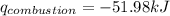
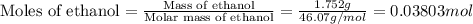
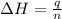
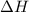 = enthalpy of combustion = ?
= enthalpy of combustion = ?