
Two aqueous sulfuric acid solutions containing 20.0 wt% h2so4
(sg = 1.139) and 60.0 wt% h2so4 (sg = 1.498) are mixed to form a
4.00 molar solution (sg = 1.213). taking 100kg of the 20% feed
solution as a basis, calculate the feed ratio (liters 20%
solution/liter 60% solution).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
Two aqueous sulfuric acid solutions containing 20.0 wt% h2so4
(sg = 1.139) and 60.0 wt%...
(sg = 1.139) and 60.0 wt%...
Questions

History, 02.12.2020 20:20



History, 02.12.2020 20:20

Mathematics, 02.12.2020 20:20

Mathematics, 02.12.2020 20:20



English, 02.12.2020 20:20

Mathematics, 02.12.2020 20:20



Mathematics, 02.12.2020 20:20


Mathematics, 02.12.2020 20:20

Mathematics, 02.12.2020 20:20




Biology, 02.12.2020 20:20

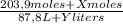 (1)
(1)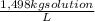 ×
×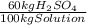 ×
×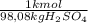 = 0,9164 kmolH₂SO₄ ≡ 916,4 moles
= 0,9164 kmolH₂SO₄ ≡ 916,4 moles

