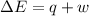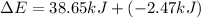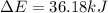Chemistry, 23.10.2019 00:30 kandigirl9990
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−3(aq)→caco3(s) co2(g) h2o(l) if 1 mol of caco3 forms at 298 k under 1 atm pressure, the reaction performs 2.47 kj of p−v work, pushing back the atmosphere as the gaseous co2 forms. at the same time, 38.65 kj of heat is absorbed from the environment. what is the value of δe for this reaction

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−...
Questions








Biology, 13.09.2019 07:20

History, 13.09.2019 07:20

Mathematics, 13.09.2019 07:20



English, 13.09.2019 07:20

English, 13.09.2019 07:20



History, 13.09.2019 07:20

Physics, 13.09.2019 07:20

Biology, 13.09.2019 07:20

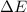 for this reaction is 36.18 kJ
for this reaction is 36.18 kJ