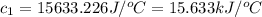Chemistry, 23.10.2019 00:00 josephbrowne9p18dit
The enthalpy of combustion of benzoic acid (c6h5cooh) is commonly used as the standard for calibrating constant-volume bomb calorimeters; its value has been accurately determined to be −3226.7 kj/mol. when 2.8161 g of benzoic acid are burned in a calorimeter, the temperature rises from 21.84°c to 24.67°c. what is the heat capacity of the bomb? (assume that the quantity of water surrounding the bomb is exactly 2250 g.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
The enthalpy of combustion of benzoic acid (c6h5cooh) is commonly used as the standard for calibrati...
Questions

Mathematics, 05.07.2019 10:00

History, 05.07.2019 10:00





History, 05.07.2019 10:00



History, 05.07.2019 10:00





Mathematics, 05.07.2019 10:00




Health, 05.07.2019 10:00

History, 05.07.2019 10:00

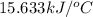
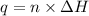
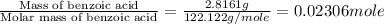
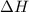 = enthalpy of combustion = 3226.7 kJ/mole
= enthalpy of combustion = 3226.7 kJ/mole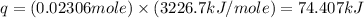
![q=[q_1+q_2]](/tpl/images/0341/5440/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0341/5440/1d50b.png)
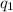 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter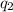 = heat absorbed by the water
= heat absorbed by the water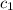 = specific heat of calorimeter = ?
= specific heat of calorimeter = ?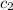 = specific heat of water =
= specific heat of water = 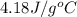
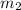 = mass of water = 2550 g
= mass of water = 2550 g = change in temperature =
= change in temperature = 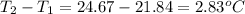
![74407J=[(c_1\times 2.83^oC)+(2550g\times 4.18J/g^oC\times 2.83^oC)]](/tpl/images/0341/5440/a2494.png)