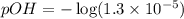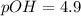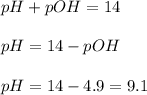Chemistry, 23.10.2019 01:00 anthonybowie99
Lithium acetate, lich3co2, is a salt formed from the neutralization of the weak acid acetic acid, ch3co2h, with the strong base lithium hydroxide. given that the value of ka for acetic acid is 1.8×10−5, what is the ph of a 0.289 m solution of lithium acetate at 25∘c?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

You know the right answer?
Lithium acetate, lich3co2, is a salt formed from the neutralization of the weak acid acetic acid, ch...
Questions

Biology, 24.03.2020 21:59


Mathematics, 24.03.2020 21:59



Mathematics, 24.03.2020 21:59



English, 24.03.2020 22:00



Chemistry, 24.03.2020 22:00

Geography, 24.03.2020 22:00





Mathematics, 24.03.2020 22:00


English, 24.03.2020 22:00

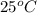 is 9.1
is 9.1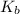 .
.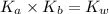
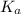 = dissociation constant of an acid =
= dissociation constant of an acid = 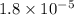
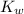 = dissociation constant of water =
= dissociation constant of water = 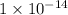
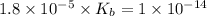
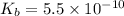
![[OH^-]=(K_b\times C)^{\frac{1}{2}}](/tpl/images/0341/6745/115e0.png)
![[OH^-]=(5.5\times 10^{-10}\times 0.289)^{\frac{1}{2}}](/tpl/images/0341/6745/5aa0e.png)
![[OH^-]=1.3\times 10^{-5}M](/tpl/images/0341/6745/43771.png)
![pOH=-\log [OH^-]](/tpl/images/0341/6745/1fac1.png)