
Chemistry, 23.10.2019 01:00 little68941
Enter your answer in the provided box. roasting galena [lead(ii) sulfide] is an early step in the industrial isolation of lead. how many liters of sulfur dioxide, measured at stp, are produced by the reaction of 5.05 kg of galena with 123 l of oxygen gas at 220°c and 2.00 atm

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Enter your answer in the provided box. roasting galena [lead(ii) sulfide] is an early step in the in...
Questions




English, 12.02.2021 21:40





Mathematics, 12.02.2021 21:40

Physics, 12.02.2021 21:40


English, 12.02.2021 21:40

Mathematics, 12.02.2021 21:40

History, 12.02.2021 21:40

Mathematics, 12.02.2021 21:40

English, 12.02.2021 21:40



Mathematics, 12.02.2021 21:40

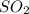 produced are 90.7 liters.
produced are 90.7 liters.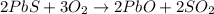
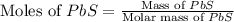
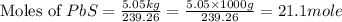
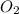 by using ideal gas equation.
by using ideal gas equation.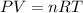
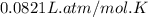
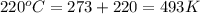
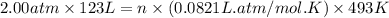
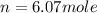
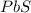
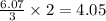 moles of
moles of 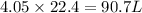 volume of
volume of 

