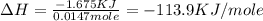Chemistry, 23.10.2019 02:30 iiMxlissaii
In a constant‑pressure calorimeter, 70.0 ml of 0.770 m h2so4 is added to 70.0 ml of 0.420 m naoh. the reaction caused the temperature of the solution to rise from 23.14 ∘c to 26.00 ∘c. if the solution has the same density and specific heat as water ( 1.00 g/ml and 4.184 j/(g⋅°c), respectively), what is δh for this reaction (per mole of h2o produced)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
In a constant‑pressure calorimeter, 70.0 ml of 0.770 m h2so4 is added to 70.0 ml of 0.420 m naoh. th...
Questions




Mathematics, 17.01.2020 19:31


Biology, 17.01.2020 19:31




English, 17.01.2020 19:31


French, 17.01.2020 19:31





History, 17.01.2020 19:31



History, 17.01.2020 19:31

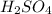 and
and 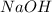 .
.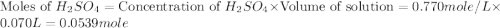
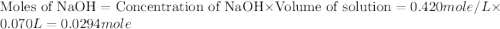
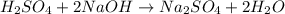
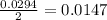 mole of
mole of 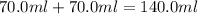
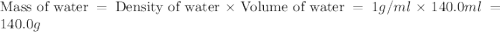
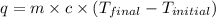
 = specific heat of water =
= specific heat of water = 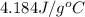
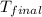 = final temperature of water =
= final temperature of water = 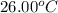
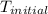 = initial temperature of metal =
= initial temperature of metal = 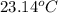
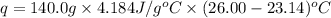
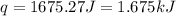
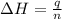
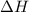 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?