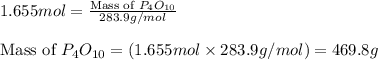Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
Phosphine, an extremely poisonous and highly reactive gas, will react with oxygen to form tetraphosp...
Questions

Mathematics, 08.06.2021 15:40

Law, 08.06.2021 15:40








Mathematics, 08.06.2021 15:40




History, 08.06.2021 15:40


Social Studies, 08.06.2021 15:40



Physics, 08.06.2021 15:40

English, 08.06.2021 15:40

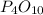 formed is 469.8 grams.
formed is 469.8 grams. ......(1)
......(1)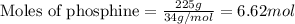
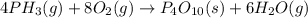
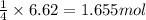 of
of