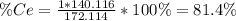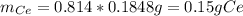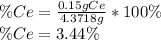Chemistry, 23.10.2019 16:50 chelseychew32
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated with excess iodate to precipitate the ce4+ as ce(io3)4. the precipitate was collected, washed well, dried, and ignited to produce 0.1848 g of ceo2 (fm 172.114). what was the weight percentage of ce (am 140.116) in the original sample?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated...
Questions

Spanish, 13.04.2021 23:10



Mathematics, 13.04.2021 23:10

English, 13.04.2021 23:10

Mathematics, 13.04.2021 23:10


Chemistry, 13.04.2021 23:10



Mathematics, 13.04.2021 23:10


Mathematics, 13.04.2021 23:10

Mathematics, 13.04.2021 23:10

Advanced Placement (AP), 13.04.2021 23:10

English, 13.04.2021 23:10

Mathematics, 13.04.2021 23:10

Chemistry, 13.04.2021 23:10

Mathematics, 13.04.2021 23:10

Chemistry, 13.04.2021 23:10

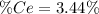
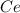 into the
into the 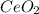 by using their respective molar masses as shown below:
by using their respective molar masses as shown below: