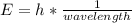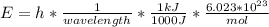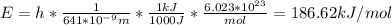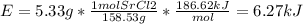Chemistry, 23.10.2019 17:00 camirialchambers17
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compound excites certain salts, which emit specific colors. strontium salts have an intense emission at 641 nm. what is the energy (in kj) of this emission for 5.33 g of the chloride salt of strontium? assume that all the heat produced is converted to emitted light. enter to 2 decimal places.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compoun...
Questions

Chemistry, 22.04.2020 15:57

English, 22.04.2020 15:57

English, 22.04.2020 15:57

Mathematics, 22.04.2020 15:57



Physics, 22.04.2020 15:58

Health, 22.04.2020 15:58


Mathematics, 22.04.2020 15:58



English, 22.04.2020 15:58


Mathematics, 22.04.2020 15:58

Business, 22.04.2020 15:58


Mathematics, 22.04.2020 15:58

History, 22.04.2020 15:58

Mathematics, 22.04.2020 15:58