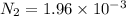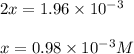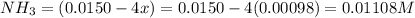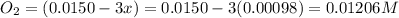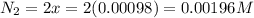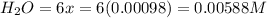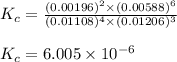Chemistry, 23.10.2019 17:00 tasnimabdallah971
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. without a catalyst, a different reaction predominates: 4 nh3(g) +3 o2 (g) ⇌ 2 n2(g) + 6 h2o(g) when 0.0150 mol of nh3(g) and 0.0150 mol of o2(g) are placed in a 1.00−l container at a certain temperature, the n2 concentration at equilibrium is 1.96 × 10−3 m. calculate kc.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. wi...
Questions


English, 19.11.2019 17:31

Business, 19.11.2019 17:31

English, 19.11.2019 17:31

Mathematics, 19.11.2019 17:31




Mathematics, 19.11.2019 17:31

Social Studies, 19.11.2019 17:31



Chemistry, 19.11.2019 17:31


Mathematics, 19.11.2019 17:31

History, 19.11.2019 17:31



Mathematics, 19.11.2019 17:31

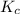 for the reaction is
for the reaction is 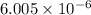
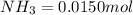
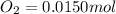
![[NH_3]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/438ea.png)
![[O_2]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/0aac8.png)
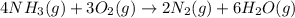
![K_c=\frac{[N_2]^2[H_2O]^6}{[NH_3]^4[O_2]^3}](/tpl/images/0342/7531/b62d0.png) .......(1)
.......(1)