Chemistry, 23.10.2019 18:30 dannyelleparker9680
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is 21/760 16 mm hg 760/21 120/75 160 mm hg submitr

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1
You know the right answer?
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of t...
Questions











Mathematics, 07.11.2019 02:31








Engineering, 07.11.2019 02:31

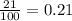
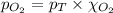
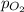 = partial pressure of oxygen = ?
= partial pressure of oxygen = ?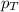 = total pressure of air = 760 mmHg
= total pressure of air = 760 mmHg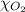 = mole fraction of oxygen = 0.21
= mole fraction of oxygen = 0.21