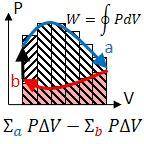
Acylinder with a moveable piston contains 92g of nitrogen. the external pressure is constant at 1.00 atm. the initial temperature is 200k. when the temperature is decreased by 64 k, by putting it in a lower temperature freezer, the volume will decrease, according to the ideal gas law. calculate the work for this process. express your answer in j. the conversion factor between liter atmospheres and joules is 101.3 j = 1 l atm. the gas constant is 0.08206 (l atm)/(mol k). molecular weight of nitrogen is 28 g/mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3
You know the right answer?
Acylinder with a moveable piston contains 92g of nitrogen. the external pressure is constant at 1.00...
Questions





Biology, 25.07.2019 17:50



Business, 25.07.2019 17:50

Chemistry, 25.07.2019 17:50



Business, 25.07.2019 17:50


History, 25.07.2019 17:50

Biology, 25.07.2019 17:50


History, 25.07.2019 17:50

History, 25.07.2019 17:50