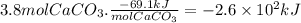Chemistry, 23.10.2019 18:00 noahdavis4650
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancient romans as mortar in stone structures. the reaction for this process is
ca(oh)2(s) + co2(g) --> caco3(s) + h2o(g) ; δh = -69.1 kj
what is the enthalpy change if 3.8 mol of calcium carbonate is formed?
(a) -18 kj
(b) -69 kj
(c) -73 kj
(d) -260 kj

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

You know the right answer?
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancie...
Questions


Physics, 17.12.2020 17:00



Mathematics, 17.12.2020 17:00

Physics, 17.12.2020 17:00









Computers and Technology, 17.12.2020 17:00

Chemistry, 17.12.2020 17:00

Social Studies, 17.12.2020 17:00


Mathematics, 17.12.2020 17:00

Mathematics, 17.12.2020 17:00