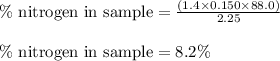The amount of nitrogen in an organic substance can be determined by an analytical method called the kjeldahl method, in which all the nitrogen in the organic substance is converted to ammonia. the ammonia, which is a weak base, can be neutralized with hydrochloric acid, as described by the equation nh3(aq)+hcl(aq)⟶nh4cl(aq) nh3(aq)+hcl(aq)⟶nh4cl(aq) if 88.0 ml88.0 ml of 0.150 m hcl(aq)0.150 m hcl(aq) is needed to neutralize all the nh3(g)nh3(g) from a 2.25 g sample of organic material, calculate the mass percentage of nitrogen in the sample.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2



Chemistry, 23.06.2019 23:30
Sodium chloride can be made as follows: 2na + cl2 → 2nacl. calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
The amount of nitrogen in an organic substance can be determined by an analytical method called the...
Questions




Biology, 26.10.2021 17:50


French, 26.10.2021 17:50





Physics, 26.10.2021 17:50








English, 26.10.2021 17:50

English, 26.10.2021 17:50

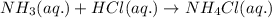
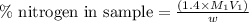
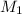 = molarity of acid used = 0.150 M
= molarity of acid used = 0.150 M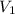 = Volume of acid used (in mL) = 88.0 mL
= Volume of acid used (in mL) = 88.0 mL