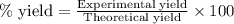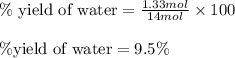Chemistry, 23.10.2019 18:00 isyssjones42
Write the balanced reaction using the fewest whole number coefficients to describe the reaction between gaseous hydrogen and gaseous oxygen. then use the information to solve the problem below: in one experiment, 14.0 moles of hydrogen and 10 moles of oxygen were reacted to produce 1.33 moles of h2o. calculate the % yield.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Write the balanced reaction using the fewest whole number coefficients to describe the reaction betw...
Questions

English, 05.10.2019 01:40

English, 05.10.2019 01:40

Chemistry, 05.10.2019 01:40


Chemistry, 05.10.2019 01:40

Health, 05.10.2019 01:40

History, 05.10.2019 01:40


Mathematics, 05.10.2019 01:40

Computers and Technology, 05.10.2019 01:40

Mathematics, 05.10.2019 01:40

Geography, 05.10.2019 01:40


Biology, 05.10.2019 01:40

Mathematics, 05.10.2019 01:40

Social Studies, 05.10.2019 01:40




History, 05.10.2019 01:40

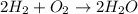
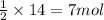 of oxygen gas
of oxygen gas