
Chemistry, 23.10.2019 19:30 griffislandon74
A5.012-g sample of an iron chloride hydrate was dried in an oven. the mass of the anhydrous compound was 3.195 g. the compound was then dissolved in water and reacted with an excess of agno3. the agcl precipitate formed weighed 7.225 g. what is the formula of the original compound?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

You know the right answer?
A5.012-g sample of an iron chloride hydrate was dried in an oven. the mass of the anhydrous compound...
Questions


Chemistry, 27.08.2019 02:40


Mathematics, 27.08.2019 02:40


Mathematics, 27.08.2019 02:40



English, 27.08.2019 02:40





Mathematics, 27.08.2019 02:40

Biology, 27.08.2019 02:40





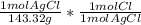 = 0.05041 mol Cl0.05041 mol Cl * 35.45 g/mol = 1.787 g Cl
= 0.05041 mol Cl0.05041 mol Cl * 35.45 g/mol = 1.787 g Cl


