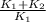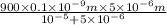Chemistry, 23.10.2019 19:00 reginapokorny
This is a reaction going on in your muscle cells right this very minute: the enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolyticpathway. it follows simple michaelis-menten kinetics: typical cellular concentrations: triose phosphate isomerase = 0.1 nmdihydroxyacetone phosphate = 5 μm glyceraldehyde-3-phosphate = 2 μm48. refer to exhibit a. what is the actual velocity of the forward reaction under physiologic conditions if km = 10 μm?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
This is a reaction going on in your muscle cells right this very minute: the enzyme triose phosphate...
Questions


Mathematics, 22.04.2021 04:50



Physics, 22.04.2021 04:50

History, 22.04.2021 04:50


Mathematics, 22.04.2021 04:50



Social Studies, 22.04.2021 04:50


Mathematics, 22.04.2021 04:50

English, 22.04.2021 04:50



Mathematics, 22.04.2021 04:50


Mathematics, 22.04.2021 04:50

Mathematics, 22.04.2021 04:50

![E + S \rightleftharpoons ES \xrightarrow[]{k_{2}} E + P](/tpl/images/0343/0177/20041.png)
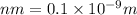
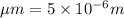
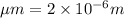
![\frac{d[P]}{dt}](/tpl/images/0343/0177/30ee3.png) =
= ![\frac{K_{2}[E][S]}{K_{M} + [S]}](/tpl/images/0343/0177/5ee12.png)
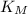 = Michaelic menten constant =
= Michaelic menten constant =