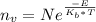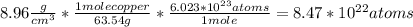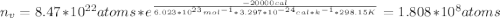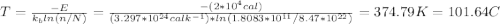Calculate the concentration of vacancies in copper at room temperature (25oc). what temperature will be needed to heat treat copper such that the concentration of vacancies produced will be 1000 times more than the equilibrium concentration of vacancies at room temperature? assume that 20,000 cal are required to produce a mole of vacancies in copper.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1
You know the right answer?
Calculate the concentration of vacancies in copper at room temperature (25oc). what temperature will...
Questions









History, 05.06.2020 04:01

History, 05.06.2020 04:01

History, 05.06.2020 04:01




History, 05.06.2020 04:01

History, 05.06.2020 04:01

History, 05.06.2020 04:01

Physics, 05.06.2020 04:01


Mathematics, 05.06.2020 04:01