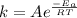Suppose that a catalyst lowers the activation barrier of a reaction from 125kj/mol to 55kj/mol125kj/mol to 55kj/mol. by what factor would you expect the reaction rate to increase at 25 °c? (assume that the frequency factors for the catalyzed and uncatalyzed reactions are identical.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Suppose that a catalyst lowers the activation barrier of a reaction from 125kj/mol to 55kj/mol125kj...
Questions

Mathematics, 11.06.2020 23:57



Mathematics, 11.06.2020 23:57


Mathematics, 11.06.2020 23:57


Mathematics, 11.06.2020 23:57



Mathematics, 11.06.2020 23:57

Mathematics, 11.06.2020 23:57




Mathematics, 11.06.2020 23:57

Mathematics, 12.06.2020 00:57

Mathematics, 12.06.2020 00:57