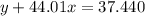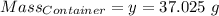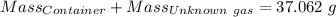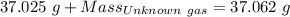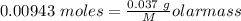Chemistry, 23.10.2019 20:30 camirialchambers17
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the same container filled with carbon dioxide at stp has a mass of 37.440 g. when filled with an unknown gas at stp, the container mass is 37.062 g. calculate the molecular weight of the unknown gas, and then state its probable identity.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1


Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1
You know the right answer?
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the...
Questions

Health, 01.04.2020 01:08

Mathematics, 01.04.2020 01:08



Mathematics, 01.04.2020 01:08



Mathematics, 01.04.2020 01:08


Chemistry, 01.04.2020 01:08

Social Studies, 01.04.2020 01:08


Business, 01.04.2020 01:08


Mathematics, 01.04.2020 01:08

Mathematics, 01.04.2020 01:08

English, 01.04.2020 01:08

Mathematics, 01.04.2020 01:08


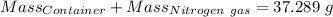
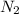 = 28.014 g/mol
= 28.014 g/mol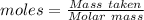
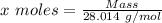
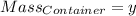
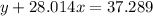
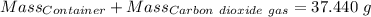
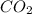 = 44.01 g/mol
= 44.01 g/mol