
Chemistry, 23.10.2019 20:00 wronganswer1026
Student determines the manganese(ii) content of a solution by first precipitating it as manganese(ii) hydroxide, and then decomposing the hydroxide to manganese(ii) oxide by heating. how many grams of manganese(ii) oxide should the student obtain if his solution contains 54.0 ml of 0.491 m manganese(ii) nitrate?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
Student determines the manganese(ii) content of a solution by first precipitating it as manganese(ii...
Questions



Mathematics, 18.03.2020 01:48

Mathematics, 18.03.2020 01:48



History, 18.03.2020 01:48



Mathematics, 18.03.2020 01:48


Mathematics, 18.03.2020 01:48


French, 18.03.2020 01:48

Mathematics, 18.03.2020 01:49


History, 18.03.2020 01:49



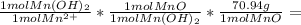 1.88 g MnO
1.88 g MnO


