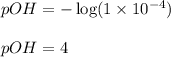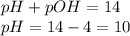Chemistry, 23.10.2019 21:00 janelisse199820
Asolution contains 1 latex: \times\: ×10−4 m oh– ions. calculate the solution ph value, and determine if the solution is acidic, basic, or neutral. math formulas: latex: ph=-\log\left[h_3o^+\right]p h = − log [ h 3 o + ] latex: poh=-\log\left[oh^-\right]p o h = − log [ o h − ] latex: ph+poh=14.00p h + p o h = 14.00 latex: \left[h_3o^+\right]\left[oh^-\right ]=1.0\times10^{-14}

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
Asolution contains 1 latex: \times\: ×10−4 m oh– ions. calculate the solution ph value, and determi...
Questions

History, 21.09.2020 02:01


Biology, 21.09.2020 02:01

Chemistry, 21.09.2020 02:01

English, 21.09.2020 02:01






Mathematics, 21.09.2020 02:01

Mathematics, 21.09.2020 02:01

History, 21.09.2020 02:01

Chemistry, 21.09.2020 02:01

English, 21.09.2020 02:01


Mathematics, 21.09.2020 02:01

English, 21.09.2020 02:01

Mathematics, 21.09.2020 02:01

History, 21.09.2020 02:01

![pOH=-\log[OH^-]](/tpl/images/0343/2369/fe336.png)
![[OH^-]=1\times 10^{-4}M](/tpl/images/0343/2369/525b6.png)