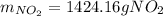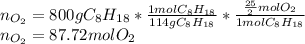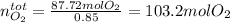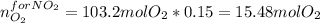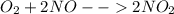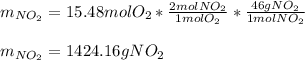The production of nox gases is an unwanted side reaction of the main engine combustion process that turns octane, c8h18, into co2 and water. if 85% of the oxygen in an engine is used to combust octane, and the remainder used to produce nitrogen dioxide, calculate how many grams of nitrogen dioxide would be produced during the combustion of 800 grams of octane.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
The production of nox gases is an unwanted side reaction of the main engine combustion process that...
Questions


Mathematics, 26.11.2020 14:00

Advanced Placement (AP), 26.11.2020 14:00

Biology, 26.11.2020 14:00


Mathematics, 26.11.2020 14:00

Physics, 26.11.2020 14:00


History, 26.11.2020 14:00


Mathematics, 26.11.2020 14:00

Social Studies, 26.11.2020 14:00



Geography, 26.11.2020 14:00


English, 26.11.2020 14:00

Mathematics, 26.11.2020 14:00

Mathematics, 26.11.2020 14:00

Mathematics, 26.11.2020 14:00