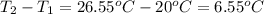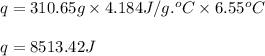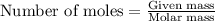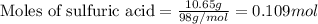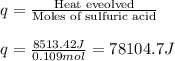When pure sulfuric acid is dissolved in water, heat is evolved. in a laboratory experiment to measure the molar heat of solution of sulfuric acid, the following procedure was followed. to a calorimeter containing 3.00 × 102 g of water at 20.00 °c, 10.65 g of h2so4, also at 20.00 °c was added. the temperature change, which was monitored by a digital thermometer with negligible heat capacity, ceased when it reached a temperature of 26.55 °c. if the specific heat of the mixture is 4.184 j g‑1 °c‑1, and the small heat capacity of the calorimeter is ignored, what is the heat evolved, per mole of sulfuric acid? show an overview of your work.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
When pure sulfuric acid is dissolved in water, heat is evolved. in a laboratory experiment to measur...
Questions






Mathematics, 19.12.2019 02:31

Mathematics, 19.12.2019 02:31





Mathematics, 19.12.2019 02:31



Mathematics, 19.12.2019 02:31



English, 19.12.2019 02:31



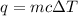
 = change in temperature =
= change in temperature =