
Chemistry, 23.10.2019 23:00 itzygutierrez2763
8.5 ml of a 0.53 m solution of the strong acid hcl was diluted with water to a total volume of 1000.0 ml at 30°c. calculate the following. (the kw at 30°c is 1.46 ✕ 10−14.)
(a) the concentration of h3o+ ions in the diluted hcl solution m
(b) the ph of the diluted solution (c) the poh of the diluted solution

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

You know the right answer?
8.5 ml of a 0.53 m solution of the strong acid hcl was diluted with water to a total volume of 1000....
Questions

Computers and Technology, 18.03.2021 01:50


Computers and Technology, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50


History, 18.03.2021 01:50




Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50



Computers and Technology, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50


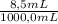 = 4,5x10⁻³ M of HCl
= 4,5x10⁻³ M of HCl

