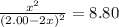Chemistry, 23.10.2019 23:00 catzdatbloadd
The concentrations of reactants and products for a chemical reaction can be calculated if the equilibrium constant for the reaction and the starting concentrations of reactants and/or products are known. part a carbonyl fluoride, cof2, is an important intermediate used in the production of fluorine-containing compounds. for instance, it is used to make the refrigerant carbon tetrafluoride, cf4 via the reaction 2cof2(g)⇌co2(g)+cf4(g), kc=8.80 if only cof2 is present initially at a concentration of 2.00 m, what concentration of cof2 remains at equilibrium? express your answer with the appropriate units.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
The concentrations of reactants and products for a chemical reaction can be calculated if the equili...
Questions

History, 02.01.2020 01:31




Mathematics, 02.01.2020 01:31

Mathematics, 02.01.2020 01:31




Social Studies, 02.01.2020 01:31


Computers and Technology, 02.01.2020 01:31








Geography, 02.01.2020 01:31

![K_c=\frac {[CO_2][CF_4]}{[COF_2]^2}=8.80](/tpl/images/0343/4238/b67f4.png)