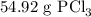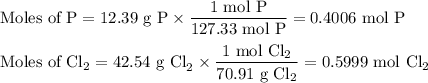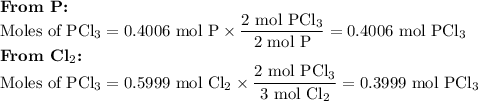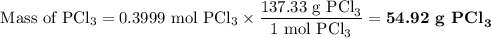Chemistry, 24.10.2019 01:00 peggycab4201
Phosphorous trichloride can be formed via a combination reaction from its elements. phosphorous is often represented by its empirical formula, p, in chemical equations, as is carbon. if 12.39 g of phosphorous is combined with 42.54 g of chlorine, what mass of phosphorous trichloride could be formed?

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
Phosphorous trichloride can be formed via a combination reaction from its elements. phosphorous is o...
Questions







Physics, 31.03.2021 08:10

Mathematics, 31.03.2021 08:10


Mathematics, 31.03.2021 08:10

Mathematics, 31.03.2021 08:10


Chemistry, 31.03.2021 08:10

Mathematics, 31.03.2021 08:10





History, 31.03.2021 08:10